Boston, MA, August 14th, 2023 - Here at Horizon Insights, we see two big trends right now in healthcare - the emergence of Artificial Intelligence, or AI platforms, and the growing prevalence and acceptance of Telehealth. Ainos, Inc. (NASDAQ: AIMD), a diversified healthcare company that develops novel point-of-care testing (POCT) medical diagnostic devices and therapeutic drug candidates for treating various diseases, is developing products incorporating both these trends into one device. Ainos has developed a technology called AI Nose designed to identify Volatile Organic Compounds (VOC), and we see this as potentially revolutionary for the future of healthcare.
You might have picked up on the fact that the name of the company, Ainos, is a play on the words AI and Nose, but you might not be familiar with exactly what VOCs are. The U.S. EPA has a nice definition of VOCs on its website. But, in short, these are gasses that are emitted from liquids or solids from things like industrial solvents, paints, refrigerants, household cleaning products, cosmetics, and other ubiquitous ingredients in our daily life that might be hazardous to our health. Air quality testing is a popular application for digital nose sensors. In fact, many countries have guidelines and regulations to monitor and control air quality for health reasons.
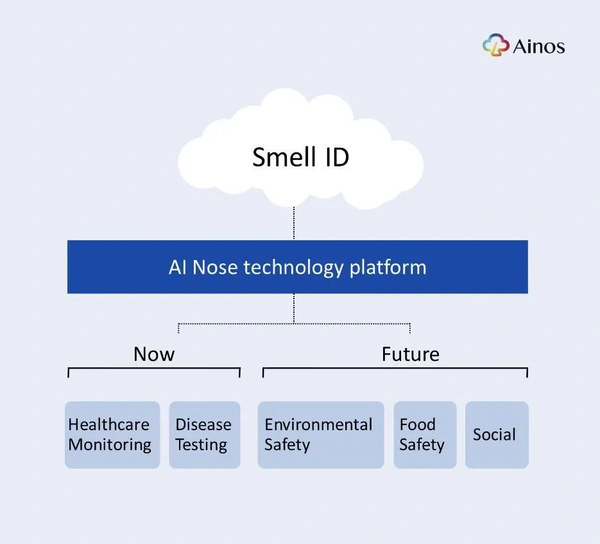
However, the human body can also release VOCs. In contrast to most environmental VOCs, human body VOCs are emitted at a very low level, sometimes produced by bacteria and other infectious microbes, and sometimes produced because the person has underlying metabolic or cardiovascular disorders. Ainos refers to the digital profiling of VOCs as the “Smell ID.”
Ainos has developed a suite of POCT products powered by its AI Nose technology that can digitally profile these VOCs, testing the user’s health condition with a handheld device. There are already many real-life clinical use cases of VOC testing. For example, the urea breath test for helicobacter pylori (H. pylori) infection is a well-established VOC clinical application. There is also an FDA-approved breath test for COVID and a breath carbon monoxide test for smokers. In Japan, there are devices that test bad breadth.
In fact, VOC analysis has been around for a long time; it has just never been as efficient as the device Ainos envisions. Conventional VOC analysis is often done at the lab with instruments including gas chromatography-mass spectrometry (GC-MS) and chemiluminescence. These systems are highly accurate and sensitive but large, complex, expensive, and clearly not practical for point-of-care/at-home use. Ainos’ AI Nose platform is designed to simplify the process and make it truly scalable through an affordable device you simply hold in your hand.
We believe there are huge long-term opportunities for using digital VOC sensing in personal healthcare. As we saw over the past two years with COVID testing, consumers are getting more and more comfortable with self-testing and remote diagnostics. We believe this new user behavior will become increasingly accommodative for Ainos’ “point-of-care technology” (POCT) products. Home self-testing and self-collection have been increasingly available for other infections, such as sexually transmitted infections.
POCT Ainos Flora
A pelvic test is the most conventional testing method for women’s vaginal health or STI. Pelvic tests are performed at the doctor’s office. It requires a trained operator, and the sampling is invasive and uncomfortable. On the other hand, there are some at-home test kits. They also require the sampling of blood, urine, or vaginal swabs, and you need to send the sampling back to the lab for processing, which will take a few days to obtain the results. None of these solutions truly offer the convenience or privacy that we believe many women desire.
The iF-award-winning Ainos Flora is designed to empower women to effortlessly, non-invasively, and quickly test their vaginal health in a point-of-care setting. Ainos Flora is designed to detect vaginal infection (including bacterial vaginosis) and common STIs (including chlamydia and gonorrhea) in minutes. The company has also designed an app to store the test result and potentially share them discreetly with the telehealth doctor. In short, it makes the process easy, fast, and discreet.
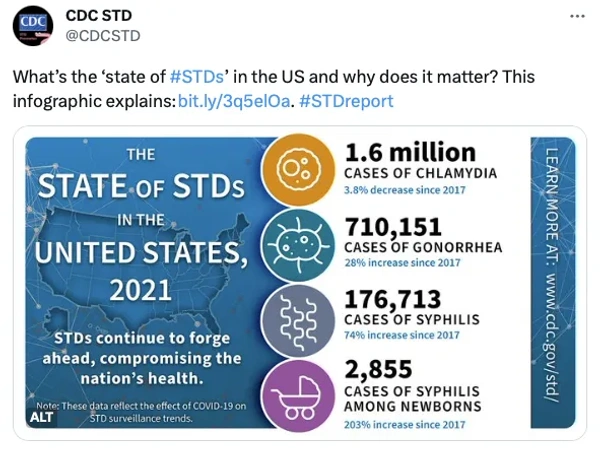
Rising STI infection is a global health issue that needs to be addressed. WHO estimated 374 million new infections of chlamydia, gonorrhea, syphilis, and trichomoniasis every year. At the same time, STI is a very private matter, and many early infections are asymptomatic. If people are not tested enough, they may unknowingly spread the infection. Ainos Flora’s convenience, speed, and privacy can encourage more testing, thereby potentially helping to manage STI infection. Ainos’ product is empowering and revolutionary for women and women’s health professionals.
It is also potentially important to young adults. STIs take a particularly heavy toll on young people. U.S. CDC estimates that youth ages 15-24 accounted for almost half of new STIs that occurred in the U.S. in 2018.
Many telehealth platforms in the U.S. have been offering STI self-test. This leads us to believe that telehealth-based STI testing service is an established business, and Ainos Flora is well-positioned to capture market share in this market.
Ainos is conducting clinical studies at several medical research centers in Taiwan, and the guidance is that we will see initial data from a proof-of-concept clinical study (NCT05557318) investigating the POCT Ainos Flora device in the fourth quarter of 2023. We believe this could be a major valuation infection point for Ainos once it validates Ainos Flora with investors.
Beyond Healthcare
In August 2023, we got the first clear glimpse of where Ainos plans to take its AI Nose technology platform beyond healthcare when the company announced a research collaboration with Nisshinbo Micro Devices Inc. (NISD) and Taiwan Inabata Sangyo Co. (Inabata) to co-develop a VOC sensing platform targeting, along with healthcare, new areas in automotive, industrial, and environmental safety. Ainos and NISD will co-develop a VOC sensing platform that leverages Ainos' AI Nose IPs, while Inabata will provide business logistics for the program and liaise between Ainos and NISD. Ainos will be compensated with an upfront development fee and per-unit fees.
We believe the three companies form a strong team, combining Ainos’ digital nose IP, NISD’s analog and electronic tech, and Inabata’s global network. NISD is a Japanese powerhouse of analog solutions serving multiple industries, including automotive, industrial, and consumer products. Inabata, the parent of Taiwan Inabata Sangyo Co., is a multifaceted trading company in four industry segments: Information & Electronics, Plastics, Chemicals, and Life industry. Inabata has a sales network in about 60 locations and has operated for more than 100 years.
As noted above, gas sensors are widely used for air quality monitoring in industrial and environmental settings. For example, testing gas leaks in factories, testing air quality in homes and offices, and some cars now test CO2 within the cabin. However, Ainos’ cutting-edge AI Nose platform, which combines digital nose sensors, AI algorithms, and the digital profiling of VOCs to create the aforementioned Smell ID, has the capacity to revolutionize air quality sensing in these scenarios. By integrating AI, Ainos can enhance traditional VOC sensing capabilities, making them more intelligent and effective - essentially giving AI a nose.
Conclusion
Ainos is capitalizing on a number of emerging trends, including the growing acceptance of telehealth and the power of AI to enhance rapid diagnostic capabilities. With the increasing rate of global infections such as COVID and influenza, consumers are becoming increasingly more comfortable with self-testing and the use of telehealth, and applications of AI-based algorithms to advance the standard of care, in healthcare and beyond. We should see updates on all these potential avenues for growth later in 2023, most notably when the initial Anios Flora study reads out in the fourth quarter, making the stock very interesting at this level.