Boston, MA, September 19th, 2023 - The U.S. Food and Drug Administration (FDA) has granted Ainos’ VELDONA Orphan Drug Designation (ODD) for the treatment of oral warts in HIV-seropositive patients. VELDONA is Very Low-Dose Oral Interferon Alpha, Ainos’ proprietary oral lozenge formulation of Interferon alpha (IFN-α) that has been extensively tested in preclinical and 68 human clinical studies, including three Phase 1, 63 Phase 2, and three Phase 3 studies, several which have been conducted in the U.S.
The FDA grants ODD to foster the development and evaluation of new medicines designed to treat, diagnose, or prevent diseases or disorders affecting fewer than 200,000 people in the U.S. ODD provides benefits including market exclusivity upon regulatory approval, exemption from FDA application fees, and tax credits for qualified clinical trials. Many of the world’s largest-selling drugs are Orphan Drugs. With ODD now in hand, Ainos plans to meet with the U.S. FDA for a pre-IND meeting, with the goal of moving VELDONA into a Phase 3 study for the treatment of oral warts in HIV-seropositive patients.
What is VELDONA?
Interferon alpha (IFN-α) is a type I cytokine secreted by immune cells that is important in regulating the immune response. It is naturally produced in response to viral infections or cancer and functions as a signaling molecule, activating other immune cells to help defend against these threats. Interferon alpha has been used for decades as a therapeutic agent to treat certain viral infections and cancers, either as a natural protein or recombinant form produced using genetic engineering techniques.
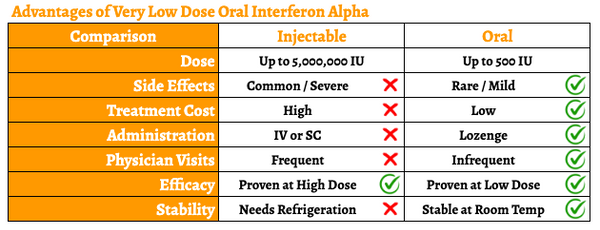
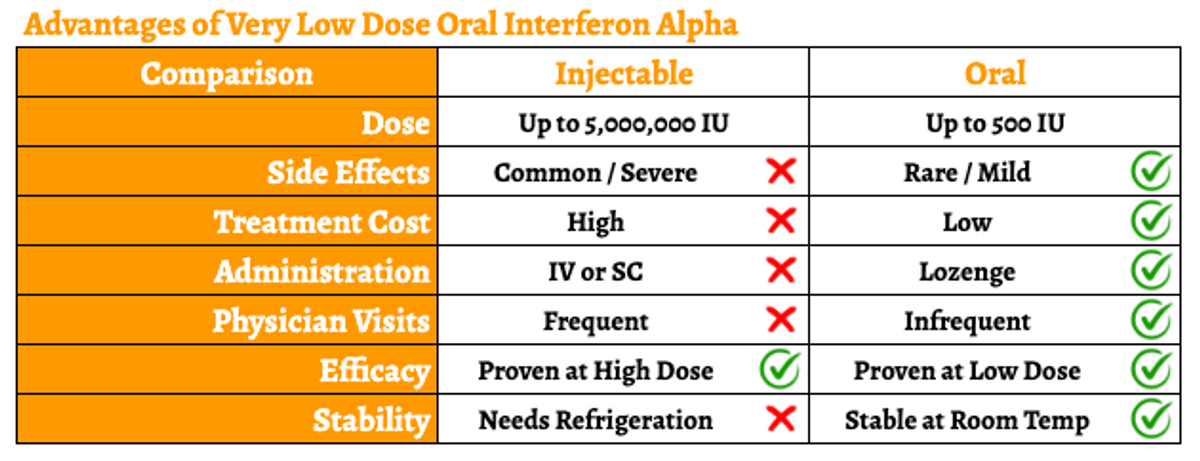
However, the drug is routinely given as an intravenous (IV) or subcutaneous (SC) injection. Because of the short half-life (approximately 2-4 hours), for the majority of indications, it must be given daily at high doses. The IV formulation must be administered in a healthcare facility, which is inconvenient. Additionally, the high systemic dose results in a range of side effects, including flu-like symptoms, muscle aches and pains, headache, nausea and vomiting, diarrhea, skin rashes, and even neurological effects such as depression or other changes in mood. These high daily doses also create undue stress on the liver and kidneys. And even though the SC formulation can be self-administered, daily injections are undesirable for most patients, especially HIV patients who manage their disease with oral medications. In short, the administration and dose-limiting toxicities of injectable high-dose IFN-α greatly limit the commercial potential for this promising drug.
VELDONA has been designed to address the need for an effective, safe, cheap, easily accessible, and self-administered IFN-alpha. The oromucosal administration of VELDONA has demonstrated retention of IFN-alpha in tissues proximal to lymphoid regions, including the posterior aspect of the nasal cavity, posterior aspect of the tongue, small intestine, and rectum. This administration route is convenient for the patient and ideal for target indications involving oral or mucosal health (including Sjögren’s syndrome, stomatitis, and oral warts).
VELDONA for Oral Warts in HIV-seropositive Patients
Ainos estimates that 24,000 Americans suffer from HIV-related oral warts. HIV destroys the body’s immune system, leaving it susceptible to opportunistic infections such as human papillomavirus (HPV), which has been established as the primary etiological factor for oral warts. In fact, research suggests up to 50% of HIV-seropositive patients may also have co-infections with HPV. As Aino’s press release notes, there is no generally accepted pharmaceutical standard of care for treating oral warts in these patients. Drugs approved to treat HIV, called highly active antiretroviral therapy (“HAART”), are ineffective against HPV. This is because HPV is not a retrovirus. Instead, HIV-positive patients with HPV co-infection turn to drugs such as cidofovir, bleomycin, cimetidine, and podophyllum, each with significant side effects and tolerability issues, or they opt for surgical removal via laser, freezing, or burning off the lesion, which can be painful and undesirable for many patients.
To date, Ainos has investigated VELDONA in nearly 6,000 patients with 16 different disease indications and/or healthy volunteers. Ainos has conducted three trial studies for the treatment of oral warts in HIV-seropositive patients. The first was an open-label pilot study involving 15 HIV-seropositive males with multiple oral warts. The second was a single-blind, dose-ranging study involving 21 HIV-seropositive subjects with multiple oral warts who received combination anti-retroviral therapy with or without PI. The third was a double-blind, placebo-controlled trial involving 80 HIV-seropositive participants on HAART, randomized to IFN-alpha (n=60) or placebo (n=20) for up to 24 weeks. The company's studies demonstrated that low-dose IFN-alpha can effectively modulate systemic and mucosal immunity without serious adverse effects, first-pass effect on the liver and kidneys, and proteolytic degradation associated with other administration routes.
We see VELDONA as a highly attractive option for HIV-seropositive patients looking to reduce the burden of oral warts. We envision the drug being taken similarly to how a patient with eczema or psoriasis might take oral corticosteroids, JAK inhibitors, or PDE4 inhibitors to clear their skin, or a patient with chronic UTIs might take antibiotics to clear their infection, or a person might take pain medications to reduce arthritic flares. When we look at the pricing for these daily oral medications, it is not unreasonable to see several thousand dollars per month. However, even at $1,000 per month, the total addressable market (TAM) in the U.S. is nearly $300 million. Outside the U.S., Ainos estimates that 1.2 million people worldwide are HIV-seropositive and suffering from chronic oral warts. Pricing outside the U.S. is less predictable, but we believe the global TAM for VELDONA for oral warts is in excess of $1 billion.
All Part of Ainos Strategic Plan
We continue to be impressed with how management at Ainos is leveraging VELDONA and the low-dose oral INF-alpha franchise. As a reminder, the company and its partners have launched VELDONA in the pet health market in Taiwan, and the guidance is that VELDONA Pet will generate $20 million in revenues in 2024. In fact, Ainos has recently announced they plan to scale up capacity with contract manufacturers to meet anticipated increasing demand.
In an effort to accelerate the development of VELDONA for human use, Ainos announced late last year that the company would pursue strategic licensing partnerships to advance the development of the drug on a global basis. The oral wart for HIV patients with ODD is an interesting asset for potential licensing partners. Beyond this indication, there are several other indications where delivering a low-dose oral IFN-α to the oral mucosa has a clear scientific rationale, including Sjögren’s syndrome, aphthous stomatitis, and radiation or chemotherapy-induced stomatitis. We look forward to the next steps for VELDONA after the planned pre-IND meeting with the U.S. FDA.